About 3 lakh tests are now being conducted per day across India. These tests include multiple types of tests like RT-PCR, TrueNat, Antigen etc. How do these tests differ from each other and what is the process of testing? Here is a detailed explainer.
India has so far conducted around 1.45 crore COVID-19 tests with the number of daily tests around 3 lakh. However, India’s tests per million continue to be among the lowest in the world, because of the huge population of over 1.3 billion. There has been a significant improvement over the past two months in the number of daily tests.
Across the world, different countries are using varied testing strategies and different types of tests. India is also using multiple types of tests to detect COVID-19 among suspect cases. Here is a detailed look at the different types of approved tests being carried out in India for COVID-19 detection.
RT-PCR Tests
RT-PCR Tests are the standard tests being conducted in India for detecting cases of COVID-19. Even across the world, RT-PCR is the most used test. RT-PCR Test implies ‘Reverse Transcription – Polymerase Chain Reaction’ test.
In this test Nasal & Throat Swabs are used to detect the presence of the virus. RT-PCR tests help in early detection of COVID-19 as this test detects the RNA of the virus, which is present in the body before the formation of any antibodies or any visible symptoms. A process called ‘Reverse transcription’ is used to convert RNA to DNA , before detecting the virus.
Process of Test:
- A sample is collected from deep inside nose and the back of the throat with a swab.
- This test requires specialised laboratory setup with specific biosafety and biosecurity precautions.
- The sample is treated with chemical solutions that remove proteins and fats, leaving only RNA present in the sample.
- The sample is sent to the laboratory for analysis, where a RT-PCR machine is used to detect the virus.
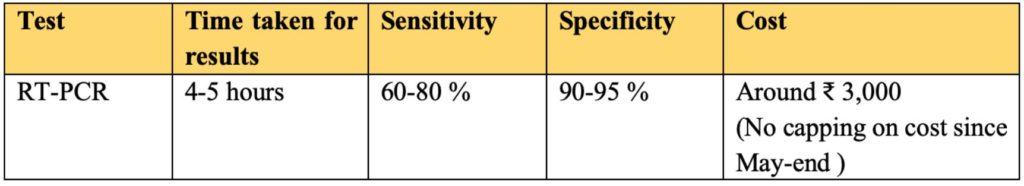
- The average time taken is around 4-5 hours from receipt of the sample to getting the result.
RT-PCR tests are more accurate and also provide the advantage of running around 90 samples in one go. Sometimes, the tests present with a false negative and hence it is conducted twice before a patient is confirmed as non-infectious.
The sensitivity of these tests is around 60-80% i.e. accuracy of finding out someone is positive and has infection (true positive rate). Whereas the specificity i.e. accuracy in identifying those without the infection is around 90-95 %.
TrueNat & CBNAAT tests
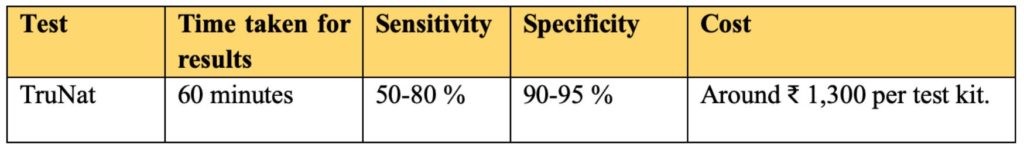
TrueNat tests have a quick turnaround time of around 60 minutes. These are commonly used for detection of Tuberculosis and HIV. TrueNat is developed by an Indian firm MolBio Diagnostics’ Pvt ltd. On 19 May 2020, ICMR approved the use of TrueNat testing for COVID-19.

The working principle of TrueNat tests is same as that of RT-PCR but uses a smaller kit. However, the challenge with these tests is that that only 1-4 samples can be run in one go. This restricts the maximum number of tests that can be conducted to around 24-48 samples per day.
Process of Test :
- The TrueNat machine that is used for the tests is a chip-based portable kit which runs on batteries.
- It detects the virus in nasal or oral swabs.
- The assay comprises of two steps. In the first step, which is an E-gene screening assay, all samples of suspect cases are tested. All negatives are considered as True Negative.
- All positives are subjected to second step of the assay. It is equipped to detect RdRp enzyme found in the RNA of the virus.
- All the samples tested positive are considered as True-positive.
Unlike RT-PCR tests, they pose minimum biosafety hazard as the viral lysis buffer that comes with the COVID-19 cartridges inactivates the virus. The minimal sample handling involved, and the close nature of the tests also limit the biohazard risk. TrueNat tests provide the scope for increase in access to testing. All the tests conducted are required to be updated on ICMR portal.
New additional strategies for testing
On 23 June 2020, ICMR issued an advisory with new additional strategies for COVID-19 testing.
- Rapid Point-of-Care (PoC) Antigen Detection Test (for diagnosis along with RT-PCR)
- IgG Antibody test for COVID-19
Rapid Point-of-Care (PoC) Antigen Detection Test
Rapid Antigen detection tests also lookout for virus similar to that of RT-PCR tests. On 14 June 2020, ICMR issued an advisory on the use of Antigen tests for detection of COVID-19.
There is no reliable antigen test across the world. The antigen test approved by ICMR is a point-of-care test, performed outside the conventional laboratory setting and is used to quickly obtain the results.
Standard Q COVID-19 Ag detection kit developed by SD Biosensor, a South Korea based company is approved by ICMR. The company has a manufacturing unit in Manesar, Gurugram, India.

Process of test
- Each test kit has an inbuilt COVID antigen test device, viral extraction tube along with viral lysis buffer and a sterile swab for sample collection.
- Only a Nasal swab is collected, which is then immersed and squeezed into the viral extraction buffer. This buffer inactivates the virus and mitigates bio safety concerns.
- After the collection, the sample is stable only for one hour. Therefore, antigen test needs to be conducted at the site of sample collection in the health care setting.
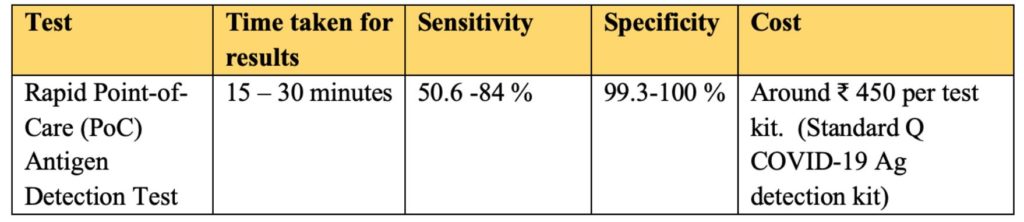
- The sample is then placed in a well of test strip. It takes around 15 minutes to interpret positive or negative result. The interpretation can be done with the naked eye without the need for equipment.
- The maximum time limit for interpretation is 30 minutes, post which it ought to be discarded.
ICMR and AIIMS have evaluated the Standard Q COVID-19 Ag detection assay developed by SD Biosensor. As per the results, the test has very high specificity in the range of 99.3% to 100% at both the sites. The Sensitivity of the test ranged between 50.6% to 84%. It is observed that higher viral load corelated with higher sensitivity.
In case the result is positive, it needs to be considered as a True Positive. However, if the result is negative, ICMR recommends that the rapid anti-gen tests needs to be confirmed with real time PCR test.

ICMR has approved the use of these tests only in:
- Containment Zones or Hot spots
- Healthcare settings
IgG Antibody test for COVID-19
IgG Antibody test is being used only for the purpose of surveillance and not for diagnosis. IgG antibodies generally start to appear after two weeks of onset of infection once the individual has recovered after infection. These antibodies are proteins produced by the body and are used by the immune system to neutralise the viruses. Therefore, the IgG test is not useful in detecting acute infection. However ICMR has advised the use of these tests for;
- Sero surveys to understand the proportion of population that is exposed to the infection. Relevant public health interventions can be planned for prevention and control of the infection based on the level of seroprevalence of the infection.
- Survey in high risk or vulnerable population to know who has been infected in the past and has since recovered.
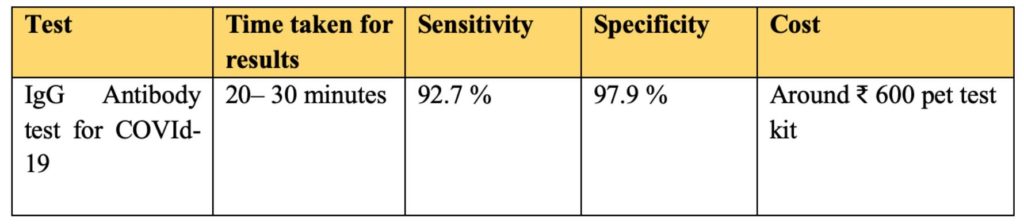
Unlike RT-PCR tests, the antibody tests require blood samples to identify antibodies for coronavirus. The results are not hundred percent reliable and may give false positives. An Anti-body tests typically gives results in 20-30 minutes. ICMR has issued a strict advisory of using only IgG based ELISA and CLIA assays for conduct of sero surveys.

India’s first anti-body testing Kit-ELISA was developed by NIV- Pune (National Institute of Virology). The test kit developed by ELISA was found as 92.7% sensitive and 97.9% specific.
The positive predictive value was 94.4% and negative predictive value was 98.14%. As per ICMR Journal, this indigenously developed IgG ELISA was found to be sensitive and specific in detection of anti-covid-19 anti bodies.
Featured Image: COVID-19 detection tests in India


