Though India opened up vaccination for all adults (those above 18 years) from 01 May 2021, the availability of vaccines is limited. Many states have not even begun administering vaccines to the 18-44 age group. But how did countries like the US & UK ensure there are enough vaccine doses for their entire population? Where did India go wrong? Here is a detailed review.
India is currently battling the second wave of COVID-19 with a record number of cases and deaths being reported each day. Globally, India now accounts for nearly one in every two cases reported in the past week and one in four deaths, according to WHO. The country’s tally of infections has crossed 2.1 crore cases (21 million). Around 2.3 lakh deaths due to the coronavirus infection have been reported, still, a low ‘Case Fatality Rate’ (CFR) compared to other countries.
Meanwhile, more than 180 countries around the world have started administering the COVID-19 vaccines, which is critical to fighting the pandemic. Vaccination is expected to help reduce the spread of the virus and bringing in herd immunity. Though vaccines may not completely prevent the infection, they can help in reducing the severity of the infection, and thereby reducing the burden on the health infrastructure, which is currently overwhelmed in many parts of India. Vaccination would also reduce mortality to a great extent. The vaccination could also help in reducing opportunities for the virus to mutate resulting in new variants.
Huge gap in vaccine administration across countries
Due to the limited availability of vaccines, most countries have come up with a vaccine strategy that prioritizes the vulnerable population such as the elderly and those with co-morbidities, and the frontline as well as healthcare workers. There is a stark gap between vaccination programs in different countries. According to the data compiled by the Our World in Data project at the University of Oxford, more than 1.19 billion vaccine doses have been administered as of 05 May 2021, which amounts to 16 doses for every 100 population. However, this is not uniform across countries. For instance, the US has administered 247.7 million vaccine doses as of 04 May 2021 whereas Zimbabwe had administered less than 0.56 million shots. The entire African continent had administered only 19.1 million doses as on the same day. India, as of 06 May 2021, has given 161.4 million doses. The COVID-19 Vaccine Intelligence Network (Co-WIN) digital platform was launched in December 2020 for recording vaccine data on a real-time basis in India.
India began vaccine administration in phases starting January 2021
According to the Union Health Ministry, India’s National Covid-19 Vaccination Strategy was formulated based on scientific and epidemiological evidence and focuses on systematic end-to-end planning with guidance from Global Best Practices, SoPs of WHO and recommendations of experts in the National Expert Group on Vaccine Administration for Covid-19 (NEGVAC). India began the administration of COVID-19 vaccines on 16 January 2021 in phases. The first phase focused on healthcare workers, and frontline workers like police, military, and sanitization staff, and those aged above 50 years. Persons with co-morbidities below 50 years of age were also included.
All adults in India are now eligible for the vaccine
The Phase-II of the vaccination drive was rolled out from 01 March 2021 and 01 April 2021 and focused on vaccinating the most vulnerable population (those aged more than 45 years). The population in this age group accounted for more than 80% of COVID-19 mortality in the country. Phase-III was rolled out from 01 May 2021 making those aged 18 years and above eligible for vaccination through the ‘Liberalised Pricing and Accelerated National Covid-19 Vaccination Strategy’. The pricing, procurement, and administration of vaccines have been made more flexible, at least on paper.

50% of vaccines produced in India will be procured by the Centre
As per the latest policy, Vaccine manufacturers in India will have to sell 50% of their produce to the Centre (GoI share) and the rest 50% (other than GoI share) may be sold to State Governments, private hospitals, and industrial establishments. Private hospitals will have to procure their supplies of the vaccine exclusively from the 50% supply earmarked for the other than GoI share. The price charged for the vaccination by private hospitals would be monitored as per the strategy. However, the policy does not stipulate any maximum price for the vaccination by the private hospitals.
Currently, only two vaccines that are being produced in India and received approval for restricted emergency use on 03 January 2021, are being administered in India; Covishield which is a version of the Oxford–AstraZeneca vaccine manufactured by the Serum Institute of India (SII) and Covaxin developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). Only one foreign-manufactured vaccine has been approved for emergency use, that too in April 2021, which is Russia’s Sputnik-V. Of the 161.4 million doses administered in India as of date, 146.1 million doses are of Covishield and 15.3 million doses are of Covaxin. Only about 31.2 million persons have received both doses.
India’s pace of vaccination has dropped starting April
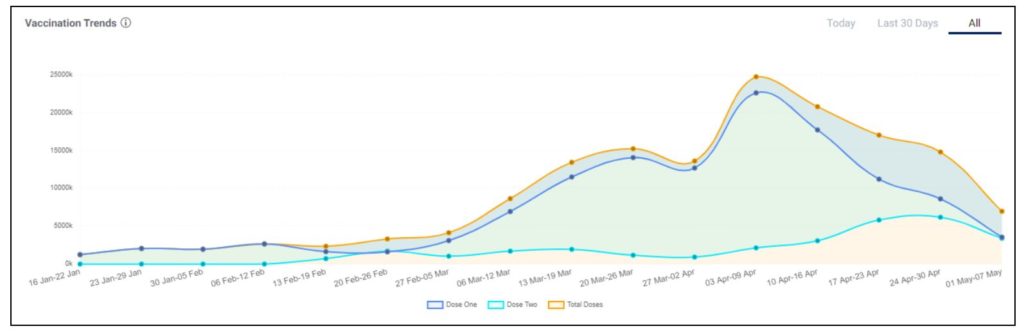
The data on vaccination drive in India shows that the total doses being administered per week went up from 4.1 million doses in the first week of March to 24.7 million doses in the first week of April. However, the weekly average has dropped to 6.97 million doses in the first week of May 2021 according to the COWIN dashboard. This drop is significant because technically 59 crore people became eligible for vaccination on 01 May 2021 as per the liberalized policy. Even with such huge demand, the pace of vaccination fell to almost a third compared to the first week of April 2021. One of the prime reasons for this could be the shortage of vaccines, as reported by states. States had to resort to pausing vaccination drives due to the shortage. As of 06 May 2021, states had only 8.9 million COVID Vaccine doses remaining with them. Close to 2 million doses were administered on 05 May 2021. It remains to be seen if the pace picks up in the coming days & weeks.
50% of population in UK has received at least one dose of vaccine
Countries like the UK and USA are carrying out their vaccination drives effectively. As of 04 May 2021, the US has administered over 247.7 million doses and the UK has administered 50.3 million doses. 51% of the population in the UK has received at least one dose while in the US, 44.1% of the population has received at least one dose. (Israel tops the list by covering 62.5% of the population). In India, only about 11% of the population has received at least one dose so far.
The difference in the pace of vaccination in countries could be due to multiple factors. One major factor is the availability of the vaccine which was ensured by countries like the US & UK by placing advanced orders starting mid-2020. The result of the difference in strategies of various countries is clearly visible in the availability of vaccines.
The US rolled out ‘Operation Warp Speed’ in May 2020
In the US, the government rolled out Operation Warp Seed as early as May 2020. It is a national program aimed at accelerating the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics involving several US Federal Government departments such as Health and Human Services (HSS), Agriculture, Energy, etc., and the private sector. US’s FDA granted approval for emergency use authorization (EUA) for vaccines developed by Pfizer-BioNTech, and Moderna in December 2020. The vaccine developed by Janssen was also approved in February 2021 but has been paused due to reports of few adverse events. The vaccination drive, first for priority groups and then for all, also began in December 2020. The US even invoked the Defense Production Act which gives the government control over private-sector industry in order to ramp up the production of materials deemed necessary for national defense.
The US would have enough doses for its entire adult population by July 2021
In May 2020, when the COVID-19 cases in the US were mounting, the US government had secured about 300 million doses of AstraZeneca’s vaccine. The Department of Health and Human Services provided $1.2 billion for this purpose. The US had also made an agreement with Moderna in August 2020 to manufacture and deliver over 100 million doses of their vaccine while clinical trials were still underway. The US government had said, ‘Moderna will manufacture the vaccine doses while clinical trials are underway. Manufacturing in parallel with clinical trials expedites the traditional vaccine development timeline’.
The US also signed an agreement for delivering 100 million doses of the vaccine with Pfizer in July 2020. Again in December, the US government placed a second order with Pfizer for another 100 million doses. All these committed orders meant that the US has already secured almost twice the number of doses required to vaccinate the entire adult population in the USA.
In fact, in February 2021, the HHS announced the purchase of an additional 100 million doses of COVID-19 vaccines from both Pfizer and Moderna. According to the press release, a total of 600 million doses of vaccine is purchased by the U.S. government from these two companies with each company is delivering 300 million doses in regular increments through the end of July 2021. In other words, the US would have enough doses to fully vaccinate its entire adult population by July 2021.
Besides, the USA Defense Department has stated that they have set aside some amount of the vaccine in cold storage as “safety stock” in case of disruptions in distribution such as weather delays or accidents. The US aims to vaccinate 70% of the adult population with at least one COVID-19 shot by 04 July, America’s Independence Day.
UK was first country to approve a COVID-19 vaccine
UK became the first country to give emergency authorization to a COVID-19 vaccine. Approval for the vaccine developed by Pfizer was given in early December 2020. AstraZeneca’s vaccine, as well as Moderna’s vaccine, were also approved subsequently within a month. Administration of vaccines also began in December 2020 in a phased manner starting with healthcare workers.
As early as June 2020, UK proactively signed a contract for 100 million doses of the AstraZeneca vaccine while the vaccine was being developed. Another deal securing access to 30 million doses of the Pfizer-BioNTech vaccine was announced in the following month which was later increased to 40 million doses in October 2020. Since May 2020, UK had secured orders from different manufacturers for a total of 400 million doses which was enough to vaccinate the entire UK population three times over. The UK, in April 2021, has announced that it has secured the order for another 60 million doses of Pfizer vaccine for the vaccine booster programme planned later in 2021.
India’s current vaccine orders not even enough to cover those aged 45 & above by July 2021
On the other hand, India has placed a net order of only 260 million doses of Covishield of which 150 million have already been supplied. The remaining 110 million doses would be supplied in the next few months (by the end of July 2021). Further, the Indian government had placed orders for a total of 80 million doses of Covaxin up to April 2021, which would be delivered by July 2021. The total vaccine availability with the Central Government by July 2021 of about 340 million would not be enough even to cover the estimated 30 crore population in the 45+ age group including the healthcare & frontline workers. Unless substantial numbers of foreign-made vaccines and other supplies are available in the coming months, the situation might not change.
Lack of Proactive planning hurting the Vaccine supplies
The available data & information clearly points to a vast difference in the approach to vaccine procurement by India on one hand and the countries like the US & UK on the other hand. Both the UK and US, which are ahead of other countries in the race to vaccinate the entire adult population had proactively placed orders for vaccines while research and trials were underway foreseeing the demand in future. Both these countries provided research grants and signed contracts for delivery of committed vaccine does in the second half of 2020. The grants & contracts enabled the vaccine manufacturers to ramp up production and deliver the doses on time. Both these countries had a deadly first wave, and through effectively procuring and administering vaccines, have managed to bring down the infection curve.
However, India, despite being home to a large vaccine manufacturing base and vast experience with national immunization programmes, did not place any advance orders that would have enabled forecasting and planning. All this meant that while the vaccination is technically open to all adults, the supply is not at par to keep up with the demand. Whether such a lack of planning would hurt India’s fight against COVID-19 remains to be seen.
Featured Image: COVID-19 vaccines


