The NPPA regulates the price of essential medicines based on the Drug (Price Control) Order of 1995 and 2013. The NPPA files cases against pharma companies found to be overcharging consumers. As per data available on the NPPA website, more than 85% of the NPPA demand of overcharged amounts on essential medicines is yet to be realized.
For more than a month, India is experiencing the second wave of COVID-19 infections. Currently, around 3.5 lakh new COVID-19 cases are being reported daily. The phenomenal increase in the number of cases has led to an increased demand for medicines and drugs that are used in the symptomatic treatment of COVID-19. Remdesivir injection is one such medication which has seen a steep increase in demand as doctors around the country have been using it for hospitalized patients. In view of this increased demand, there are multiple news reports of it being sold in the market at much higher prices than the stipulated price.
While the authorities are taking steps to crack down on the black market of such essential drugs, there are also policies aimed at keeping the prices of such drugs under control to make them accessible. Recently, following the intervention of the National Pharmaceutical Pricing Authority (NPPA), manufacturers of Remdesivir have announced a voluntary reduction of the prices of this injection.
In this story, we take a look at the role of NPPA in regulating the prices of medicines & drugs in India and the action taken by the authority against violations as per the existing regulations.
NPPA fixes the Ceiling price for medicines as per the provisions of DPCO
The National Pharmaceutical Pricing Authority (NPAA) was established as an independent body on 29 August 1997, as per the decision taken by the Cabinet Committee reviewing Drug Policy of 1994. It is attached to the office of the Department of Pharmaceuticals (DoP) in the Ministry of Chemicals and Fertilizers.
The key functions of NPPA are to act as a regulator for the pricing of drugs and to ensure availability & accessibility of medicines at affordable prices and to enforce the provisions of the Drugs (Price Control) Order (DPCO).
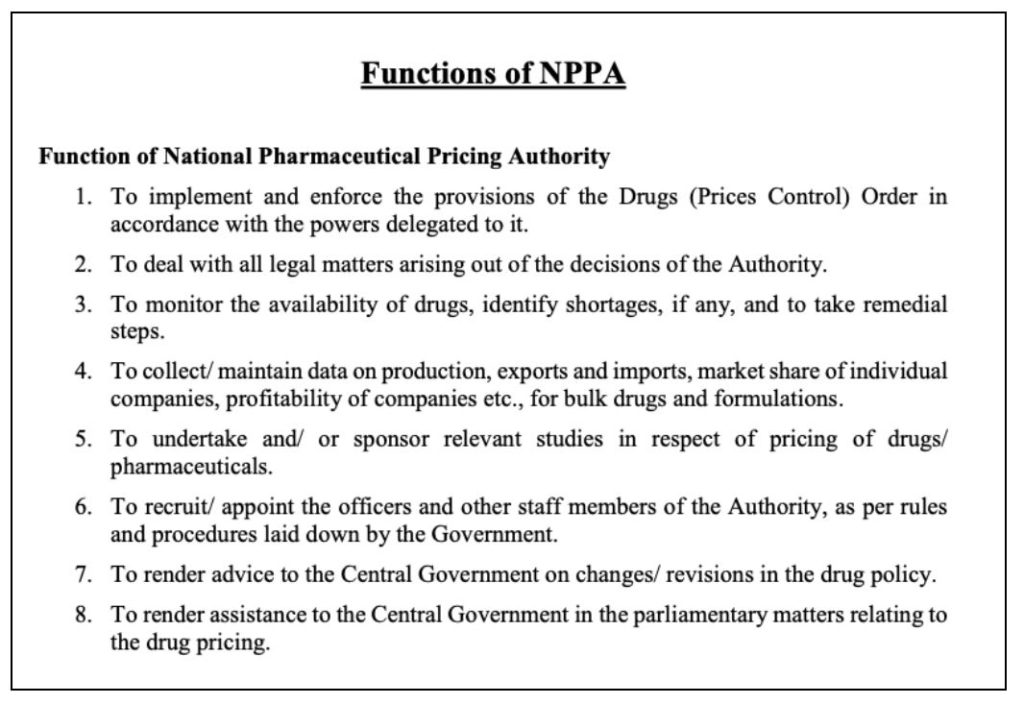
DPCO 2013 has brought additional drugs under the pricing control ambit compared to DPCO 1995
The latest DPCO which is under effect is the 6th Amendment of DPCO 2013 i.e. Drug (Prices Control) Amendment Order, 2019. One of the key aspects of this amendment is that the government would consider market-based data to fix the prices of the drugs.

This amendment is the latest among the various amendments made to the original Drugs (Price Control) Order, 2013. The DPCO, 2013 came into effect from 15 May 2013 and empowered NPAA to regulate the prices of 348 essential drugs. Prior to DPCO (2013) regime, the DPCO 1995 regulated the pricing of 74 bulk medicines. As per DPCO (2013), the drugs that are in the National List of Essential Medicine (NLEM) are monitored and controlled by NPPA. Apart from including additional drugs under the ambit of DPCO (2013), a few other changes were also made compared to DPCO (1995).
- DPCO (2013) is governed by NPPA based on NLEM.
- As per DPCO (1995), once the prices were fixed, they cannot be changed as per the act. However, DPCO (2013) introduced Simple Average Price (SAP). Based on SAP the highest prices of the drugs can be lowered taking into consideration the margins.
- In DPCO (1995), the Ceiling & Non-Ceiling prices of the drugs were not specified, while DPCO (2013) contains such provisions.
- DPCO (1995) applied a ‘Cost plus pricing’ formula, wherein companies were allowed to make a nominal profit over the manufacturing costs. As highlighted earlier, DPCO (2013) has introduced a market-based pricing mechanism. The new formulation has also benefitted the drug makers, creating a win-win for the government & manufacturers.
Hence as per DPCO (2013), NLEM assumes prominence as all the drugs that are enlisted in it are part of NPPA’s price regulation. The latest NLEM was notified in 2015 and implemented in 2016.
Around Rs. 8.18 thousand crores are pending to be collected for Overcharging cases initiated by NPPA
Responding to a question in the Lok Sabha, the government provided details regarding the cases initiated by NPPA against drug manufacturing companies for overcharging. The NPPA issues notice to the companies who are found to be overcharging the consumer.
As per the information provided in the Lok Sabha:
- NPPA has initiated a total of 2116 overcharging cases since its inception.
- Of these, 881 overcharging cases are pending, which amounts to a total of Rs. 8.18 thousand crores. These cases were initiated by NPAA under various DPCO regimes including 1979,1987, 1995 & 2013.
- 324 cases are under litigation in the courts. The amount under litigation comes to a total of Rs. 6.55 thousand crores.
Before DPCO (2013) regime came into effect, a total of 926 cases booked under DPCO (1995) during the period 1997-2013. A total of Rs. 3.02 thousand crores of overcharged amount (including interest) was demanded for the overcharged drugs. Of this, more than 80% which is around Rs. 2.55 thousand crores were still outstanding.
Since 2013-14, i.e., since DPCO (2013) was introduced, cases were initiated under both DPCO (1995) and DPCO (2013). By the end of 2019-20, there are a total of 2099 cases were registered under the different DPCOs with the amount demanded valued at Rs. 6.42 thousand crores. Of this, Rs. 5.44 thousand crores are still outstanding. This is as per the information provided in the year-wise summary of the outstanding cases report (as of September 2020) available on the NPPA website. In 2020, a total of 13 cases were initiated as per the information available.
As per the information provided in the report, Status of Overcharging Cases, as of September 2020, CIPLA pharmaceuticals has around 50 cases pending under various stages. Among other pharma companies with a higher number of pending cases, Sun Pharma has 42 pending cases followed by Dr. Reddy’s Labs with around 30 pending cases.
Only about 15% of the amount demanded by NPPA has been collected so far.
As the data in the Year-wise summary of the Outstanding Cases Report indicates, a major portion of the amount demanded for overcharging is yet to be realized. In fact, the data shows that only about 15% of the demand amount is collected since 1997.
The information relates to both the regimes i.e., DPCO, 1995 and 2013. Both the overall value of the amount due to be collected, as well as the duration of the dues raises serious questions on the efficacy of the overall process of initiating the cases against the companies that are overcharging and the subsequent recovery process.
In its answer in Lok Sabha regarding the status of recovery, the government mentioned that the recovery of the overcharged amount is a continuous process. While NPPA pursues the cases challenged in the court, those that aren’t challenged in courts are referred to the Collector/District magistrate for recovery of the overcharged amount.

However, looking at the amount due under litigation and pending, the recovery process seems quite ineffective. The main purpose of cases initiated by NPPA is to impose penalties on companies and to deter them from overpricing in the future. But such long delays would defeat the purpose of ensuring the companies abide by the regulations.
Featured Image: Price of essential medicines


