A post claiming that Bharat Biotech has received permission from the central government to launch the vaccine for COVID-19 and that the vaccine will be available in the market from 15 August 2020 is doing rounds on social media. The claimants are posting the letter from ICMR to Bharat Biotech to support their claim. Through this article lets fact-check the claim made in the post.

Claim: Vaccine for COVID-19 by Bharat Biotech will be available in the market from 15 August 2020.
Fact: The ICMR letter only mentions that they envisage to launch the vaccine by 15 August 2020, and not make the vaccine available in the market. Bharat Biotech founder had said that if the clinical trials of Covaxin go well and meet the highest safety and efficacy standards, and if the regulators approve of them, it can be available for mass use by early 2021. Hence the claim made in the post is FALSE.
If we closely observe the letter which is going viral, it is said in the letter that they envisage to launch the vaccine by 15 August 2020, after all clinical trials, however the final outcome will depend on the cooperation of all clinical trial sites involved in this project.

When we ran a search on internet using the keywords ‘ICMR Bharat Biotech Vaccine’ we found several news articles reporting ICMR-Bharath Biotech to launch COVID vaccine by 15 August 2020, these articles quoted the letter written by ICMR to Bharat Biotech. These news articles can be seen here and here. Hence, we can say that the letter is a genuine one.

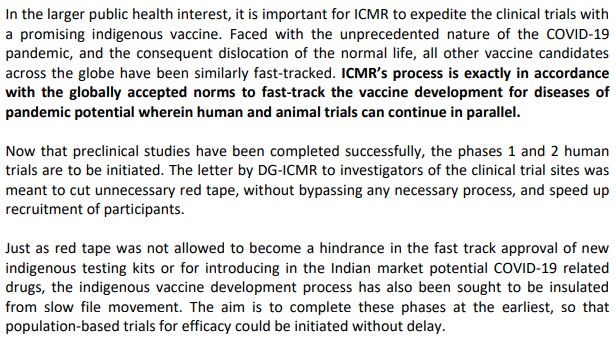
As ICMR attracted criticism for their haste regarding the launch of the vaccine, they clarified through a press release that ‘ICMR’s process is exactly in accordance with the globally accepted norms to fast-track the vaccine development for diseases of pandemic potential wherein human and animal trials can continue in parallel‘. Also, in the press release it said their intention is to that to make sure red tape and slow file movement do not become hindrance to the development of the vaccine.
Also, talking to Mint, an ICMR official on condition of anonymity said “Our internal communication is being misinterpreted. We only said that we envisage to have a vaccine by 15 August 2020, and it is not a deadline. We have not said that we will launch a vaccine by then. The process can be expedited but the vaccine still will have to undergo all safety clinical trials”.

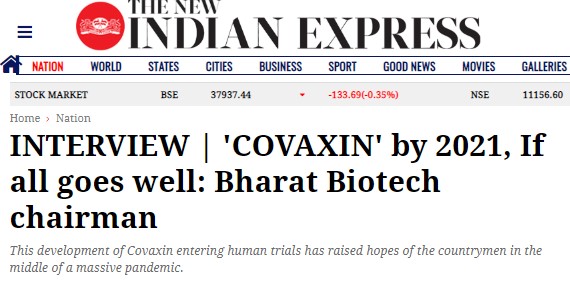
Also, in an interview with New Indian Express, Dr Ella, Bharat Biotech founder had said that ‘if the clinical trials of Covaxin go well and meet the highest safety and efficacy standards, and if the regulators approve of them, it can be available for mass use by early 2021’.
To sum it up, ICMR letter asking Bharat Biotech to fast track approvals is misinterpreted as permission to launch the vaccine for public use and is being shared as proof that the vaccine will be available in the market from 15 August 2020.
Did you watch our new video?


