Many countries around the world have begun administering the public with COVID-19 vaccine developed by multiple companies like Pfizer, Moderna etc. Each of these countries has come up with a prioritization strategy to administer the vaccine to the most vulnerable groups as the availability is limited. Here is a look at such strategies around the world and in India.
Generally, it requires many years of research, testing, and trials for a vaccine to be rolled out for public use. During all these phases, the possible side effects both short-term and long-term are studied. However, in the wake of the COVID-19 pandemic, scientists and pharmaceutical companies worldwide have worked at jet speed towards developing a safe and effective vaccine to tackle this public health emergency of international concern. All these activities in the development of a vaccine have been condensed into less than 12 months.
61 vaccines are in clinical development stage
According to WHO’s latest landscape of novel coronavirus candidate vaccine development, there are a total of 61 vaccines in the clinical development stage and 172 in the pre-clinical evaluation stage as of 22 December 2020. After about a year’s efforts across the globe, countries have begun giving emergency approvals for the widespread public use of COVID-19 vaccines.
Various strategies have been devised by nations in order to ensure that the vaccines first reach the most vulnerable groups such as frontline healthcare workers who are at the highest risk of exposure, aged persons, and those with comorbidities, owing to the limited availability. A sufficient number of vaccines to immunize the entire population will not be available immediately and hence there is a need for a prioritization strategy.
FDA has given Emergency Use Authorization for Moderna & Pfizer Vaccines
Recently, the Food and Drug Administration (FDA) of the USA granted approval for emergency use authorization (EUA) for the coronavirus vaccine developed by New York-based Pfizer and the German company BioNTech. EUA is also granted for a vaccine developed by Moderna & NIAID. EUA is a mechanism under which the FDA has the power to permit the use of unapproved medical products for preventing serious conditions, provided certain criteria are met such as unavailability of approved and adequate alternatives.
Many countries have approved and begun administering vaccines
Pfizer was the first vaccine candidate to make public the vaccine’s efficacy. In early December, UK became the first country to give emergency authorization to a COVID-19 vaccine, the one developed by Pfizer. Bahrain was the second country to approve the Pfizer vaccine. Bahrain, like the USA, has given EUA. Other countries where the vaccine has been authorized for emergency use are Canada, Mexico, 27 member states of the European Union, Middle Eastern countries like Oman, Qatar, Kuwait, Saudi Arabia, and the UAE, Serbia, and Chile. Singapore is the first Asian country to give regulatory approval.
USA and Canada have given EUA to the vaccine developed by Moderna. Similarly, Sputnik V developed in collaboration with the Russian health ministry was approved in Russia as early as August 2020. However, there were few concerns raised on the clinical trial process. Currently, the vaccine has been given approval for emergency use in Argentina and Belarus. The vaccine developed by CanSino has been given limited use approval in China. Bahrain, UAE, and China have also given approval for the China-based Sinopharm vaccine. India has not yet approved any of the vaccines. The Central Drugs Standard Control Organization is responsible for vaccine approval in India. The CDSCO has sought additional data from those that have applied for emergency authorization.
Not only have the vaccines been approved, but countries have also started administering their use. Recently, the CDC has announced that the USA has passed a milestone by giving the first dose of vaccine to over 1 million people using the two authorized vaccines which were discussed earlier. UK, Israel, Mexico, and other countries that gave emergency approval have also begun their vaccination drives.
Price of vaccine varies across countries
As per reports, the price of the vaccine in the European Union (EU) according to a deleted tweet of Belgian Minister is as follows-
- Oxford/AstraZeneca: €1.78 (£1.61).
- Johnson & Johnson: $8.50 (£6.30).
- Sanofi/GSK: €7.56.
- Pfizer/BioNTech: €12.
- CureVac: €10.
- Moderna: $18.
Another report stated that the cost of the Pfizer vaccine in the USA would be $19.50 per shot which would be higher than that in the UK. Meanwhile, countries like France, Norway, and Japan have announced that the COVID-19 vaccine will be free for all residents. In few other countries, it is being given free for those who are recommended the vaccine.
Multiple strategies to ensure equitable distribution of vaccine
The distribution of the vaccines is being done on the basis of strategies devised by respective governments. The USA has rolled out Operation Warp Seed under which several US Federal Government departments such as Health and Human Services (HSS), Agriculture, Energy, etc., and the private sector are collaborating. The plan was announced as early as September when vaccine trials revealed promising results. The Advisory Committee on Immunization Practices (ACIP) of USA recommended that both healthcare personnel and residents of long-term care facilities be offered the COVID-19 vaccine in the initial phase of the vaccination program. The USA Defense Department has stated that they have set aside some amount of the vaccine in cold storage as “safety stock” in case of disruptions in distribution such as weather delays or accidents.
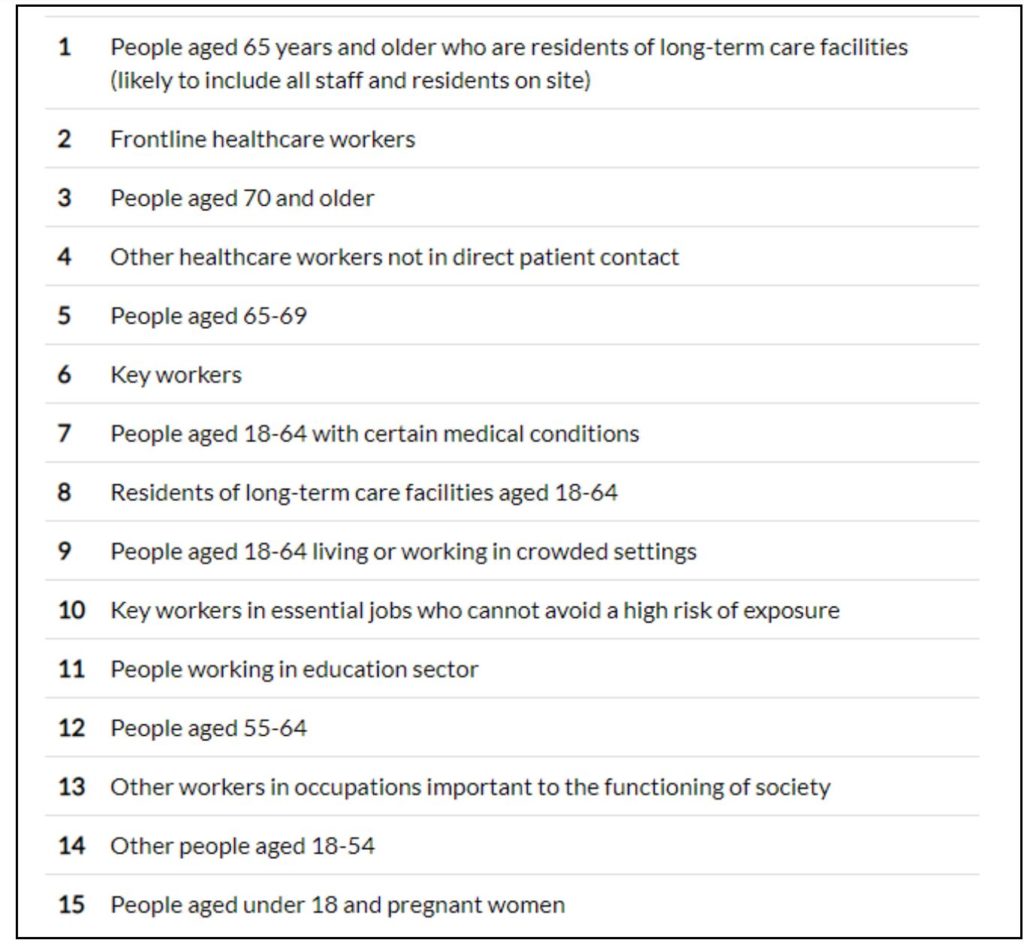
The UK’s priority list is to cover residents in ‘care homes for older adults’ and their carers, those aged above 80 years, frontline healthcare and social workers, and then those aged 75 years and above, and in descending order of age, covering all aged 50 years and above. The motive behind covering the said age groups is to cover around 99% of the preventable deaths due to the coronavirus.

Unlike in the UK, Ireland has prioritized those aged 65 years and above who are residents of long-term care facilities, followed by frontline healthcare workers. The next priority has been given to persons aged 70 years and above. Ireland’s strategy caters to not just persons on the basis of age but also those who are vulnerable and are at a heavy risk of exposure due to the sector in which they work. The list is not only comprehensive but also provides the rationale and ethical principles behind including each of these categories of individuals.
Government of India has not released a comprehensive priority list as yet
In India, the Government released recently a list of FAQs on the COVID-19 vaccine. These FAQs do not go into details of who will be vaccinated. The document states that based on the availability of vaccines, the government has selected priority groups based on risk. The first group includes healthcare and frontline workers. Persons aged over 50 years constitute the second group. Taking the vaccine will be voluntary. But it will be mandatory that people register themselves online for the vaccination and also bring a photo ID card with them.
The government in a press release in early December stated that over 30 crore people have been line-listed for the vaccine including healthcare workers, frontline workers like police, military, and sanitization staff, those aged above 50 years and with co-morbidities.
Government has to overcome other roadblocks
Besides the issue of the supply of vaccines, a few other challenges for the government would be the availability of adequate logistics and storage facilities for these vaccines. For instance, the vaccine developed by Pfizer has to be stored at ultra-cold sub-zero temperatures making the transportation & subsequent storage a logistical challenge. In addition, since the vaccine development took place at an extraordinary pace, there will be many people skeptical of the efficacy and wary of side effects. Educating the public and ensuring the informed decisions is another challenge that needs to be addressed.


