Close to 50 private labs are now authorized to conduct COVID-19 tests. But how are samples collected and tests conducted for COVID-19? Here is a detailed explainer.
As India enters the second week of lockdown, more than 1200 cases of COVID-19 have been reported across the country including 32 deaths, according to the Ministry of Health’s dashboard as of 9.30 PM on 30 March 2020.

The rapid spread of disease across the globe has challenged the healthcare systems of many countries. In an earlier story by Factly, it was observed that testing in India was low as compared to other countries which could be one of the reasons for a proportionately lower number of cases reported in India. In order to boost testing, the Indian Council of Medical Research (ICMR) which is India’s apex biomedical research body, has started approving private labs who are now authorized to carry out testing in addition to the government labs.
Private labs are being given approval to conduct COVID-19 diagnosis tests
As on 30 March 2020, ICMR had given approval to 49 private laboratories who can conduct COVID-19 diagnosis tests. This includes 10 laboratories in Maharashtra, 8 each in Delhi and Telangana, 6 in Tamil Nadu, 5 in Haryana, 4 in Gujarat, 2 each in Karnataka, West Bengal and Kerala and 1 each in Odisha and Uttar Pradesh. This is in addition to the 123 government laboratories which have been approved and supported by ICMR as on 30 March 2020. Thus, in total, so far, more than 170 labs have been given approval by ICMR for testing people for COVID-19.
However, the number of approved laboratories for COVID-19 testing is bound to increase in the coming days as more laboratories apply for permission and the availability of testing kits improves.
It has to be noted that private labs are not allowed to conduct tests for COVID-19 without the approval of ICMR. Only those private laboratories which hold National Accreditation Board (NAB) for Testing and Calibration Laboratories’ accreditation for real time Polymerase Chain Reaction (PCR) testing for RNA virus and also possess all testing material will be given approval by ICMR. The structure of the coronavirus would be shared by ICMR once the approval is provided.

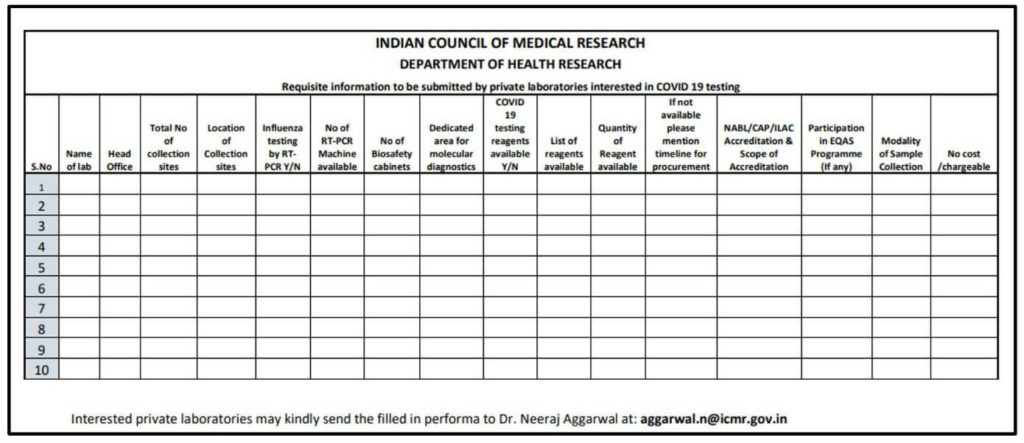
The following details must be sent to ICMR, by laboratories interested to perform COVID-19 diagnosis tests in order to get approval. The details include location, number of collection sites, available materials and machines, accreditation scope, and cost for test.
Who must get themselves tested?
In the beginning, the government had a testing strategy which included testing mostly the those individuals with history of foreign travel. This included asymptomatic individuals with history of foreign travel only if they show symptoms. Also included in the testing pool were contacts of the laboratory confirmed positive cases.

On 20 March 2020, the government modified the testing strategy. As of now, the government has restricted tests to only symptomatic individuals who
- Have a record of international travel in the last two weeks
- Have had contact with those who have been tested positive
- Healthcare workers
- Are patients hospitalized with Severe Acute Respiratory Illness (Fever and cough/shortness of breath)
Further, those asymptomatic persons who are in the high-risk category or have had direct contact with those tested positive, must be tested after almost one week since the contact was made.

What sample is collected?
Samples are collected from people who are supposed to get tested. According to World Health Organization and US Centers for Disease Control and Prevention (CDC), the following are the respiratory samples that need to be collected for COVID-19 diagnosis tests. It is mandatory that biosafety and biosecurity precautions are taken for sample collection.
From the lower respiratory tract,
- Tracheal Aspirate – with the help of a tube inserted into the lungs, a sample of 2-3 mL of the sample is collected in a sterile dry container
- Sputum – Mucus is collected from the person through coughing
From the upper respiratory tract,
- Nasopharyngeal swab /oropharyngeal swab – with the help of cotton swab, samples are taken from inside the throat or nose
- Nasal Aspirate– a solution is first injected into the nose and gently suctioned to get the sample. About 2-3 mL of sample is collected in a sterile dry container.
In addition to these, blood sample can also be collected for testing for virus. Furthermore, urine, stool, and even material from autopsy in case of dead persons are collected for tests and research.
ICMR recommends private laboratories to collect samples from suspected persons at their homes since this helps in reducing human contact while travelling and in collection centers. Further, at the time of sample collection, the government ID proof of residence and contact details of the suspected patient is also collected.
What happens to the collected samples?
Up on ensuring that the collected samples are properly packed taking into consideration the required precautions as the virus is highly contagious, the sample must be safely transported to the approved lab for testing.
For the detection of viral infections, there are two types of tests that are mainly performed- one is genetic and the other is serological. Genetic tests are used to identify infections which are currently active in the sample whereas serological tests can also help trace past infections. PCR Test, abbreviation for Polymerase Chain Reaction Test, is a genetic test that is performed to identify the presence of SARS-CoV-2 virus. For this, the RNA in virus is converted to DNA by the process of PCR. Reverse Transcriptase Polymerase Chain Reaction or RT PCR Test is also performed for qualitative study of gene expression. In short, there are two tests performed, one which looks at all the coronaviruses present and the other which looks for only SARS-CoV-2.
It should be noted that only those US FDA EUA/CE IVD approved kits which have been approved by DCGI and notified to ICMR should be used. ICMR has also established fast-track mechanism for non-US FDA EUA/CE IVD approved kits.
What happens once tests are done?
All labs must send the samples of those tested positive to the National Institute of Virology, Pune strictly adhering to the biosecurity and biosafety precautions. The negative samples must be destroyed within a week of collection. All biomedical waste produced must be disposed of carefully as per the Biomedical waste management rules, 2016. The samples must be discarded, and swabs should be in sealed biohazard bag that contains 2% Lyzol or 5% freshly prepared hypochlorite solution.
No sample should be shared with other organizations. It is also illegal to culture the virus from the sample or use the same for other purposes. This has been strictly prohibited and any violation will result in legal action.
What is the cost for testing?
While ICMR recommends that the sample testing should be done for free or at a subsidized rate, the cost of test has been capped at Rs. 1,500 for screening suspected cases and Rs. 3000 for confirmation test. That is, the maximum price for testing samples should not exceed Rs. 4,500 as per the recommendations of National Task Force.

One should note that the details given in the story are as per the latest details available as on 30 March 2020. The guidelines and protocols may undergo changes based on the severity of the pandemic in India.
Featured Image: COVID-19 testing in India



1 Comment
FICO scores is 780 now and it posted on all reporting agencies with the service of 760 Plus Credit SCore : It also works if u have a VERY GOOD credit score and need inquiries, collection accounts, charge offs or debt etc removed. A few months ago I read about them and just last month I decided to give them a try, today I can boost of a perfect credit profile. All thanks to 760 Plus Credit Score. I strongly recommend them if you desire to fix Credit Score. Here is the contact:
Email: 760pluscreditscore @ gmail dot com