The race to get everyone vaccinated has just begun with UK, USA & Canada issuing EUA for the Pfizer- BioNTech COVID-19 vaccine. Data maintained by the Duke University indicates that more than 50% of the confirmed purchases are by High-Income countries. The vaccination in India could begin soon with three different vaccine candidates having applied for EUA.
A second wave of COVID-19 infection is sweeping across the USA with over 2 lakh cases being reported daily. Meanwhile, the second wave experienced in the major European countries appears to have subsided with fewer numbers being reported barring Germany. Meanwhile, based on the earlier trends and timelines, it is estimated that the second wave of COVID-19 infection could soon hit countries like India & Brazil, which are among the countries with several COVID-19 cases during the first wave.
The World Health Organization (WHO), and governments across the world have been advocating the use of masks, social distancing, sanitizing, etc. to contain the spread of COVID-19. While these measures are in place, countries across the world have been waiting for a vaccine with bated breath.
The expectation is that a vaccine could inoculate the population from COVID-19. The Center for Disease Control & Prevention (CDC), emphasizes the benefits of getting a COVID-19 vaccine which would protect individuals against the infection and help contain the pandemic.
Multiple vaccine candidates are being developed world-wide and are at different stages of development process.
As per WHO’s latest Draft landscape of COVID-19 candidate vaccines, 52 candidate vaccines are in clinical evaluation of which at least 13 are in phase-3. An update on the New York Times states that 7 vaccines are approved and are in the stage of early/limited used or approved for full use.
United Kingdom has begun its vaccination drive with the first-person receiving the vaccine developed by Pfizer last week. The United States Food & Drug Administration (US FDA) also issued the first emergency use authorization (EUA) for the vaccine developed by Pfizer-BioNTech. It is expected that the inoculation will begin in a day or two in the USA. Even the Indian drug regulator has sought more data & information from three different vaccine candidates that have applied for EUA.
However, the vaccine strategy followed by the countries around the world is not uniform. Few of the richer countries have been proactive in sourcing vaccines while many other poor & middle-income countries are lagging behind. Access to vaccines, distribution infrastructure, healthcare infrastructure, economic status etc. are few of the factors that influence the approach of these countries.
High income countries have already reserved more than half of the confirmed purchase of Vaccine doses
The imbalance among the countries in procuring the COVID-19 vaccine is evident from the consolidated information provided by Launch & Scale Speedometer, an initiative of Duke University’s Global Health Innovation Center.
In a recent press release, they have pointed out that the low-income countries would have access to fewer vaccine doses as a majority of the vaccine doses to be made available are already reserved by the rich countries.

Vaccines generally take multiple years to develop with extensive trials being undertaken to ensure safety and desired outcomes. However, in the context of the global pandemic, many of the vaccine candidates have been fast-tracked and as highlighted earlier, few of them are in the final stages to be rolled out while few others have been given EUA.
However, the major challenge is to manufacture enough doses to meet the global demand. The current manufacturing capacity is limited and there is a race among the countries to gain first access to the vaccine doses being manufactured.
Launch & Scale Speedometer reports that various countries have already finalized contracts to the tune of nearly 7.2 billion doses even though none of the vaccines are yet approved for public use except for EUA in a few countries. There are another 2.4 billion doses that are under negotiation.
High income countries have reserved a major chunk of these vaccine doses
As per the latest information available as of 11 December 2020, High-income countries have made transactions for nearly 3.91 billion doses. Meanwhile, Lower Middle-income countries and Upper Middle-income countries have reserved 1.6 billion and around 1 billion doses respectively. None of the low-income countries have got into any contracts for at least the initial doses expected to be manufactured.
COVAX is an initiative aimed towards equitable distribution of COVID-19 vaccine
The volume of the vaccine doses blocked by a few of the wealthier nations can be used to inoculate its citizens multiple times. For example, the USA has made deals for around 1 billion doses which are around 3 shots on average per person. The same can be said of Canada & U.K.
Meanwhile, the lower-income countries could be in a situation where the available number of vaccine doses would not be enough to inoculate even a major section of the population. To ensure equitable distribution of the COVID-19 vaccine when it is made available, an initiative called COVAX was launched as part of the ‘Access to COVID-19 Tools (ACT)’ Accelerator. COVAX is a coordinated effort by Gavi- the Vaccine Alliance, Coalition for Epidemic Preparedness Innovations (CEPI), and the WHO.

All the participating countries, irrespective of their income status would have equal access to these vaccines once they are developed. This initiative is especially helpful for Low-income countries that would otherwise not be able to afford the vaccines. The initial effort is to procure at least 1 billion doses by end of 2021 so that at least the high-risk vulnerable sections of the population gain access to the vaccine.
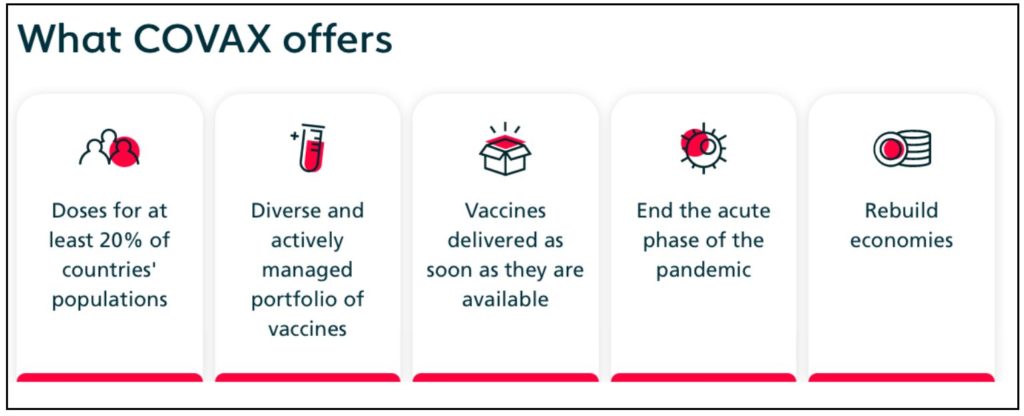
While this is a much-needed initiative, the direct bi-lateral deals of self-financed rich countries with the manufactures may prove to be hampering the equitable efforts. While the USA is not part of COVAX, U.K and Canada are part of the COVAX alliance but have also entered into large-scale individual deals. Such deals could create challenges in ensuring access of vaccines to lower-income countries. With no direct deals, the low-income countries would be reliant on the 20% population support that COVAX would provide.
India has the largest advanced confirmation of COVID-19 vaccine doses thanks to its manufacturing capacity
Different countries are using multiple strategies to gain access to the COVID-19 vaccine. While the higher income countries were able to get into advance deals with manufacturing companies thanks to their economic strength, few of the middle-income countries can gain leverage due to their other strengths. Peru, for example, has been able to negotiate deals for vaccine in exchange for the clinical trials they are offering.
Meanwhile, India and Brazil, two of the largest middle-income countries were able to negotiate substantial deals thanks to their vaccine manufacturing capacity.
As per the data provided by Launch & Scale Speedometer, India has already done advanced deals for 1.5 billion doses. This is only topped by the advance deals made by the European Union. Of this, 500 million doses are for Oxford University developed the vaccine and 1 billion doses are for Novavax. While there is no confirmation on any payment by the Government of India for these deals, the claim is based on the manufacturing companies based in India.
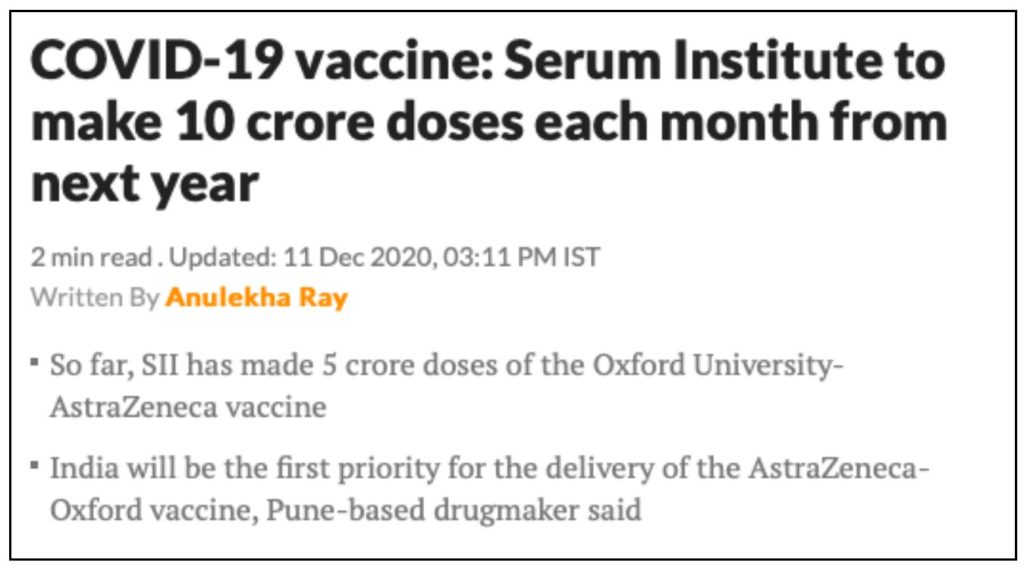
Pune-based Serum India Institute (SII) would be manufacturing Oxford University Vaccine in India. As per the update provide by SII, they have already manufactured 5 crores doses of this vaccine and plan to manufacture 10 crores does per month starting from 2021. India would be the first priority for the Oxford vaccine. This vaccine would be called Covishield in India.
The 1 billion doses of Novavax as claimed in Launch & Scale Speedometer, is thanks to the agreement between SII and Novavax to manufacture COVID-19 vaccine in India.

However, this vaccine is still under development and as per the latest update in November 2020, it was granted fast-track designation by the US FDA.
Apart from Oxford University’s Covishield, two more vaccines are under consideration in India. One being developed by Pfizer and another is the indigenously developed vaccine – Covaxin. Covaxin is being developed by Hyderabad-based Bharath Biotech in collaboration with the India Council of Medical Research (ICMR).
As per the available information, Pfizer has applied for Emergency Use Authorization (EUA) with the Drug Controller General of India (DGCI). It ought to be noted that the vaccine currently being administered in the U.K is the one developed by Pfizer. As per the update from ICMR, indigenously developed Covaxin has received approval from DGCA and currently is in phase-3 of clinical trials.

Apart from these vaccines, other vaccines are also under trial. One of them is the Sputnik vaccine from Russia. Dr. Reddys Laboratories in collaboration with the Gamaleya Research Institute of Russia is conducting phase-2 of clinical trials. Another vaccine is being developed by Biological-E ltd, which is under phase 1/2 of human clinical trials.

Therefore, the higher number indicated for India in the Launch & Scale speedometer report is thanks to the vaccine manufacturing companies based in India. India will be given priority over these vaccines. However out of the 1.5 billion doses, only the 500 million doses of Covishield is an immediate reality, provided there is emergency approval from the government. The 1 billion doses of Novavax could take more time as it is still under development.
The government of India puts plans in place for vaccinating the first 30 million Indians
Procurement of the COVID-19 vaccine is the first step towards vaccinating the population. With the large scale capacities of the vaccine manufacturers in India, we are well-positioned to get access to the vaccine as and when they are approved.
However, the major challenge would be in logistics involving the distribution of the vaccine. The efficacy, suitability, and sustainability of a particular vaccine candidate is a choice which the country has to make. For example, Pfizer has a higher efficacy rate at 95 % compared to Oxford University’s Covishield. However, in terms of infrastructure needed, Oxford and other Indian developed vaccines have an edge as Pfizer’s vaccine has to be kept at -70 degree Celsius, which could pose a logistical challenge to ensure the vaccine reaches even the remotest parts of the country.
In a first formalized step towards Vaccine distribution, the Government of India has recently announced its plan through a Press release by the Ministry of Health and Family Welfare.
Highlights of the plan announced by the government include:
- A new digital platform for COVID 19 vaccination delivery – ‘CO-WIN’ is being developed.
- 30 crore population to be prioritized for vaccination. This includes 1 crore Healthcare workers, 2 crore front line workers followed by 27 crores of Prioritized Age group (those above 50 years and those with co-morbidities).
- Developing Cold Chain Infrastructure (85 thousand + vaccine storage equipment at 28,897 cold chain points across the country for the first 3 crore doses for healthcare & frontline workers).
- Services of 1.54 lakh ANMs (Auxiliary nurse midwives) to be used for COVID -19 vaccination.
In the press interaction, it was also updated that the National Expert Group on Vaccine Administration for COVID- 19 (NEGVAC) was formed in August’2020 to aid in prioritization of population groups, procurement, and inventory management, vaccine selection, vaccine delivery & tracking mechanism.
With the initial plan set in motion, it is to be seen how it would be implemented at the ground level. Creating awareness about the vaccine, identifying any side effects and immediate responses, developing infrastructure for facilitating the delivery across the country would be the key in ensuring the vaccine reaches every person. Given the manufacturing capabilities, delivery infrastructure, the development process of the vaccine, etc. it ought to be understood that it could take more than a year for the vaccine to be available for everyone. WHO’s ‘Roadmap for prioritizing population groups for vaccines against COVID-19’ can act as a guide.
While the government maintained that ‘Every single Indian who needs to be vaccinated will be vaccinated’, it can look at various studies that suggest the vaccination for only a certain part of the population to be enough to achieve immunity against COVID-19. This would be beneficial especially for a highly populated country like India.


